Update: Since this article’s publication, the Center for Disease Control and Prevention (CDC) has updated its guidance regarding the use of face masks to help prevent the spread of COVID-19. These changes have been reflected within the article. Always check the CDC website for the most up-to-date information.
The novel coronavirus, known as COVID-19, has now spread to 160 countries and resulted in over 7,500 deaths. Due to limited hospital space and a shortage of medical supplies, healthcare systems around the globe are facing difficulties treating all of those infected. Unfortunately, during this time of great need some safety mask suppliers are profiting by selling masks falsely bearing approval labels from the National Institute for Occupational Safety and Health (NIOSH).
NIOSH sets U.S. standards for safety masks and lists all NIOSH-approved masks online.
A Sayari investigation found that some of the suppliers selling counterfeit “NIOSH-approved” masks identified by the Center for Disease Control and Prevention (CDC) are local subsidiaries or partners of global safety equipment providers. The findings not only reveal companies engaging in a deceptive business practice, but also expose the risk they pose to the general public who relies on medical-grade masks and the companies that might source their products.
The COVID-19 Pandemic
On Mar. 11, 2020, the World Health Organization’s (WHO) director general declared COVID-19 a pandemic. The response from citizens has been one of relative panic. Fear-driven shopping sprees for hand sanitizer, toilet paper, and masks has led to cases of price gouging and contributed to the aforementioned supply shortages, which is adversely affecting those on the frontline of the pandemic—healthcare workers.
According to the CDC, the most effective way to slow the spread of COVID-19 is through social distancing and basic hygienic practices. In addition, while earlier guidelines advised that only those who are sick or caring for the sick wear safety masks, on Apr. 3 2020, the CDC began recommending that Americans wear face masks in public.
What Are NIOSH-Approved Masks?
It is no surprise that companies are falsely claiming that their masks are “NIOSH-approved.” The NIOSH air filtration rating is the U.S. standard for classifying a face mask’s ability to filter particulate matter and it is widely used by top-tier safety companies, including 3M. NIOSH will only approve safety masks that filter a minimum of 95% of particulate matter, hence NIOSH’s “N95” approval.
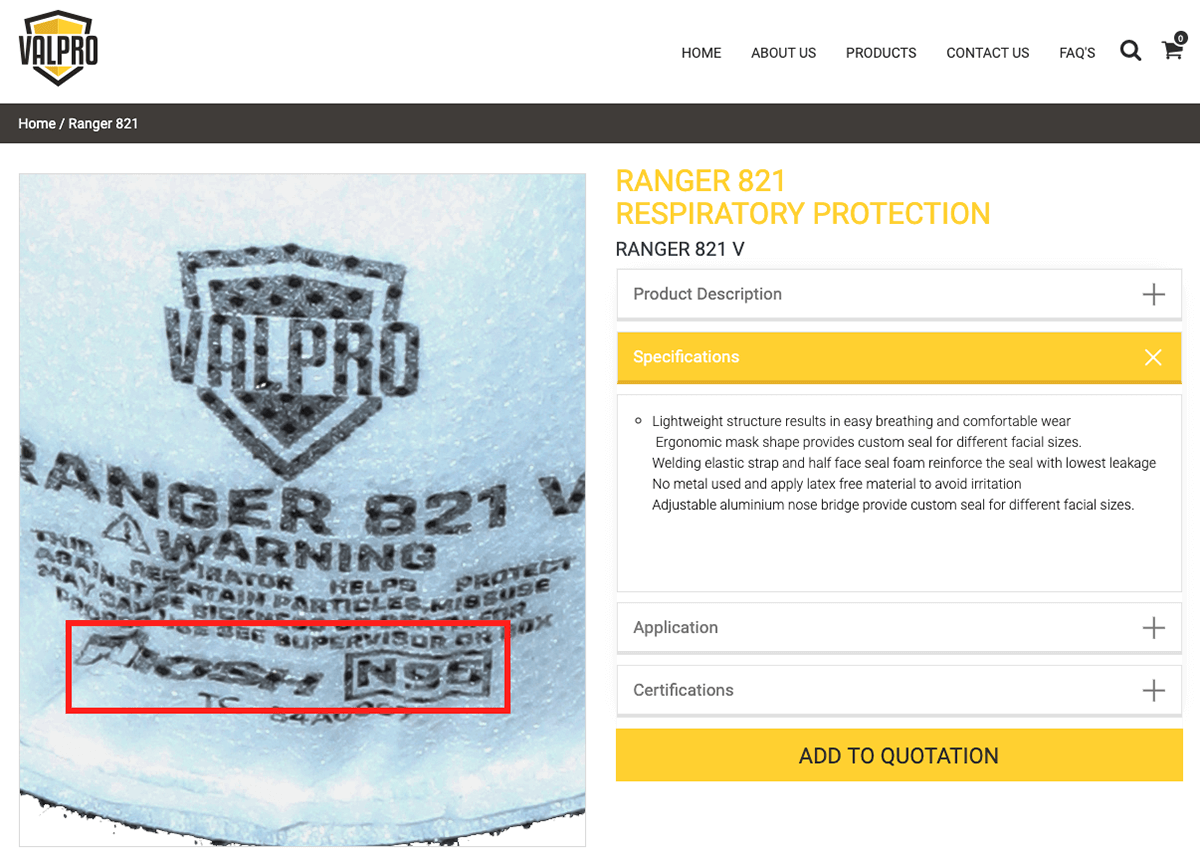
Fig 1. A screenshot from UAE-based Valpro, falsely using the NIOSH N95 approval rating.
UAE Company Linked to Global Supplier
According to the CDC’s list of counterfeit safety/respirator masks, UAE-based Valpro markets falsely-labeled “NIOSH-approved” masks. Valpro is a safety equipment supplier linked to Midas Safety—a global safety apparel supplier based in Ontario, Canada. Valpro’s website uses an @midasafety.com email domain and the same phone number listed on the official Midas Safety UAE website. Moreover, the Jebel Ali Free Zone Commercial Register lists the country of origin for Midas Safety (ME) as Canada, further confirming their link to the Canada-based Midas Safety.
A particularly troubling aspect of this finding is that Midas Safety is marketing the falsely-labeled masks only in jurisdictions with less regulatory enforcement. The Midas Safety websites for Saudi Arabia, UAE, Sri Lanka, Oman, Pakistan, China, and Bangladesh all list falsely-labeled “NIOSH-approved” masks, while their Canada, UK, and Germany websites do not.
Some companies mislead customers, such as Singapore-based Pasture Pharma which markets a falsely-labeled “NIOSH-approved” mask but carries NIOSH approval for several other products. However, NIOSH does not list either Valpro or Midas Safety (ME) anywhere on its “NIOSH-Approved Particulate Filtering Facepiece Respirators” list.
Falsely-labeled “NIOSH-approved” masks are cause for concern and should be taken seriously by public officials. They not only pose a risk for individuals who use them, but for companies that might rely on them to protect their employees. During a pandemic like COVID-19, workers operating on the frontlines, especially in medicine, need to know that the masks they are wearing will offer protection for themselves and those they are helping.
For more information on counterfeit respirators and misrepresentation of NIOSH-approval, please visit the CDC website.



