At least two of the companies have sent over 400 shipments of various pharmaceutical products valued above $700 thousand globally between 2016 and 2019, according to commercial trade data provided by Panjiva.
Given the companies’ connection to a convicted drug smuggler, any one of the entities in the network are at high risk of illegally importing pharmaceuticals into the United States or other jurisdictions.
U.S. authorities target India-based drug smuggling network
On July 7, a federal judge sentenced Jeetendra Harish Belani to time served and three years of supervised release after he pleaded guilty to conspiracy to import schedule II and schedule IV controlled substances, conspiracy to smuggle misbranded drugs, and conspiracy to commit money laundering.
The sentencing comes after a December 2019 guilty plea, whereby Belani admitted that he, along with other co-conspirators, ”imported into the United States various drugs available only by prescription, including tapentadol, a schedule II controlled substance, as well as tramadol, carisoprodol, and modafinil, all schedule IV substances,” according to a U.S. Department of Justice (DOJ) press release. The drugs were then sold to “individual customers and online re-shippers in the United States,” according to the indictment.
Belani imported the drugs via an India-based pharmaceutical company — LeeHPL Ventures Private Limited — where he is listed as a director, according to the India Ministry of Corporate Affairs (MCA).
He also admitted to working with two U.S. nationals to smuggle etizolam, a drug often used to treat anxiety and insomnia but that also carries risk for abuse and overdose. Belani worked with co-conspirators to smuggle the drug into the U.S. where it was illegally resold on a website — www.etizy.com. Etizolam has not been approved by the Food and Drug Administration, and thus cannot be “imported, distributed, or prescribed in the United States for use as a drug,” according to DOJ.
Belani attempted to conceal the nature of the shipments from U.S. officials at ports of entry. He and his co-conspirators used false customs declarations and divided large shipments into smaller quantities that “were shipped to multiple addresses to help ensure delivery and avoid interception by United States customs authorities,” according to the DOJ press release.
Finally, co-consiprators sent “tens of thousands of dollars” in payments to foreign accounts controlled by Belani.
India’s emerging role as a source of illicit synthetic drugs
The case of Jeetendra Belani is indicative of a growing trend, namely the emerging role of India as a key source of illicit synthetic drugs, along with licit drugs sold illegally.
India boasts one of the largest pharmaceutical industries in the world, ranking third in terms of overall volume and first in generic drug production. The country has recently become known for its exports of tramadol, a synthetic opioid that is less potent than fentanyl. While fentanyl has signaled a new phase in the opioid epidemic in the U.S., the use of tramadol — once considered a safer opioid — has led to a growing public health crisis in parts of Africa and the Middle East.
India also appears to be an emerging source of fentanyl and fentanyl precursors, according to an unclassified report published by the Drug Enforcement Administration (DEA) in January.
The majority of fentanyl, fentanyl analogues, and fentanyl precursors continue to be sourced from China. However, seizures in recent years of fentanyl and/or chemical precursors sourced from India point to the country’s emerging role as a source country. This is likely attributed, at least in part, to greater enforcement in China and in the Hong Kong Special Administrative Region in recent years to control the manufacture and export of fentanyl and fentanyl precursors, according to the DEA report.
The Belani case, along with other recent drug trafficking cases, point to the growing role of India-based drug trafficking networks in illegally supplying the United States with other types of opioid and non-opioid prescription medications.
In September 2019, U.S. authorities arrested eight individuals who were allegedly involved in a scheme to import and distribute large quantities of “misbranded tramadol and other drugs” from India, according to a DOJ press release.
The drugs were shipped from distributors in India to the U.S. via “the U.S. mail and other commercial couriers.” Upon receival, the defendants allegedly repackaged the drugs in a warehouse in New York, and then mailed them to customers throughout the country.
Belani’s pharmaceutical network in India
Belani serves as a director of the following India-based companies:
- Castor Lifecare Private Limited
- Maan Medex Private Limited
- Deneb Healthcare Private Limited
- LeeHPL Ventures Private Limited
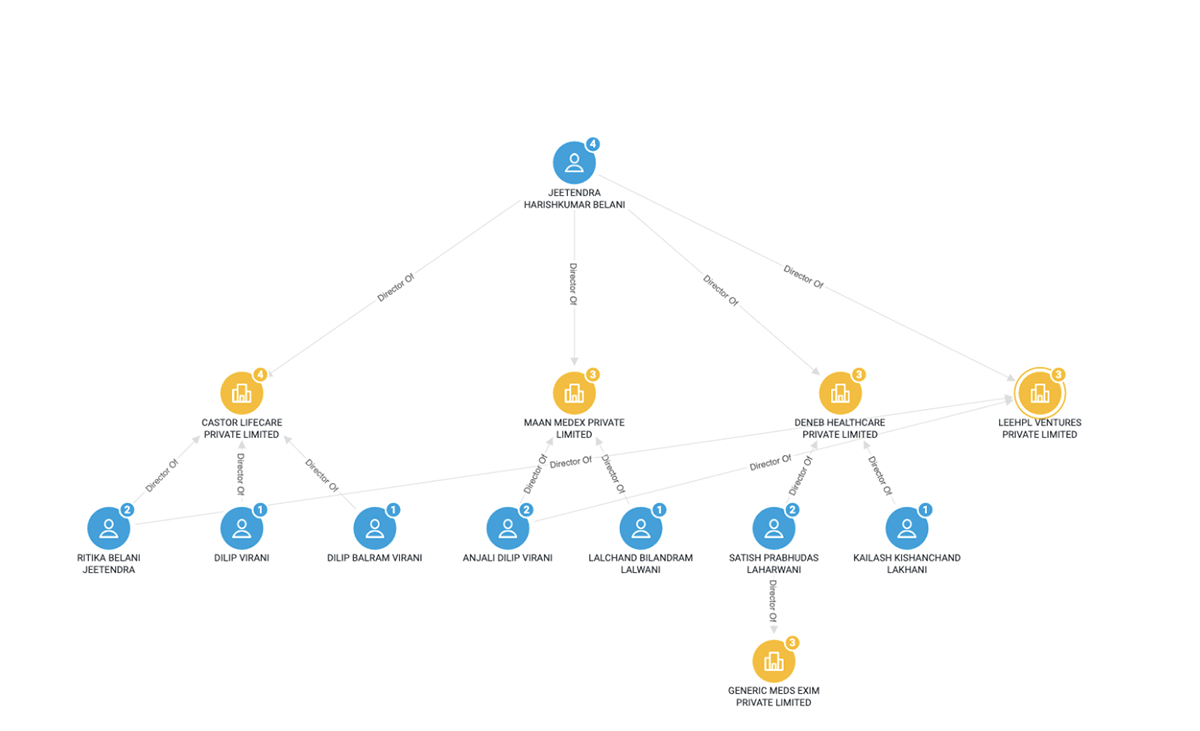
Fig.1: Sayari Graph snapshot of Jeetendra Belani’s corporate network in India.
Trade data suggests that at least two companies connected to Belani — LeeHPL Ventures Private Limited and Maan Medex Private Limited — have a history of exporting pharmaceuticals internationally.
Between 2016 and 2019, Maan Medex sent at least 108 shipments of various pharmaceuticals valued at $127 thousand — including some of the same prescription medications Belani illegally imported and sold in the U.S., according to commercial trade data from Panjiva.
Bills of lading show that the shipments were sent to Singapore, Bahrain, Costa Rica, and Iraq, with 104 of the 108 shipments sent to Singapore.
Maan Medex’s 104 shipments to Singapore were addressed to one of two courier service companies — Singapore Post or DHL Ecommerce Singapore Pte., Ltd.
What’s more, the bills of lading also show that several shipments included tapentadol, carisoprodol, modafinil, and etizolam — some of the same pharmaceuticals that Belani illegally imported and sold in the U.S.
Additionally, Maan Medex’s shipping patterns have been similar to those of LeeHPL Ventures. Between July 24, 2018 and June 10, 2019, LeeHPL Ventures sent at least 287 shipments containing various pharmaceuticals to Singapore. The value of the shipments amounted to over $505 thousand, and Singapore Post or DHL Ecommerce Singapore were listed as the consignees on all but two of the shipments.
Apart from Singapore and the U.S., LeeHPL Ventures has sent at least 15 shipments of pharmaceuticals to other countries, including Iraq, Bahrain, the UK, Moldova, Lithuania, Venezuela, and Brazil.